Research Article 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Response of Injured Lung to Qiapi 1© Treatment in Rats Exposed to Penta-Valent Arsenic
*Corresponding author: Arturo Solís Herrera, Human Photosynthesis© Research Centre, Sierra del Laurel 212, Bosques del Prado Norte, Aguascalientes, Mexico.
Received: April 18, 2022; Published: May 02, 2022
DOI: 10.34297/AJBSR.2022.16.002206
Abstract
The main function of lungs is to perform the exchange of CO2 with the alveolar air from atmosphere. At the respiratory membrane, where the alveolar and capillary walls meet, gases move across the membranes, with the carbon dioxide exiting from bloodstream. It is through this mechanism that blood carbon dioxide, the waste product of cellular metabolism, is removed from the body. The particularities of normal gas exchange require ventilation, perfusion, a normal alveolar surface, and energy. The respiratory membrane is highly permeable to gases; the respiratory and blood capillary membranes are very thin; and there is a large surface area throughout the lungs. There are many toxic compounds in the environment that cause hampering pulmonary defense against bacterial and viral infection in humans and animals during acute or chronic exposition, however, their mechanisms of suppression of pulmonary host defense are not well understood. It is likely that the combination of epithelial cell injury and inhibition of macrophage function may be responsible for the suppression of pulmonary host defense. In this study we expose tri-valent arsenic to different groups of Wistar rats. There are a control group, an exposed group, and a third group that simultaneously was exposed and at the same time treated with a novel therapeutic agent (QIAPI 1©). At the end of the study of four months, the animals were sacrificed, and inner organs studied. In this report we are describing the histological lung results.
Background
One of the most important compounds in nature is carbon dioxide (CO2 which plays a central role in photosynthesis, respiration, and acid-base balance. Although CO2exists in several different chemical forms, at least two of these forms, i.e., HCO3 and CO22-, cannot easily diffuse across most cell membranes [1]. HCO3- and CO22-, often dominate the total CO2movements through the aqueous solutionbathing membrane. It is thought that less oxygen may be inhaled when breathing is relaxed while sleeping. Less oxygen may also be inhaled due to lung disease, causing the PAO2to become lowered. Unlike CO2 oxygen requires a high-pressure gradient to diffuse.
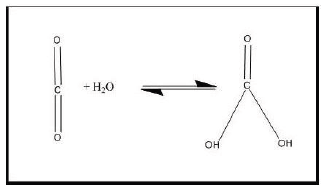
The Partial Pressure of Oxygen (PO2) in room air is 160mmHg, PN2 600 mm Hg; the PCO2 is 0.296mm Hg. The mean experimental value of DO2 in pure water is about 2 × 10-5 cm2 s-1 at 20 °C and is sensitive to the temperature. The value of O2 diffusion coefficient in pure water at 25 °C and 1.0 atm O2 pressure is varied from 1.9 to 2.3 × 10-5 cm2 s-1. The value of CO2 diffusion coefficient in pure water at 25 °C and 1.0 atm of pressure 1.91 × 10-5 cm2 s-1. However, the presence of carbonic anhydrase enzyme (CA) causes a 10 to 100-fold stimulation of the CO2 flux (Figure 1).
The value of diffusion coefficient of carbon dioxide at 20 °C (68 °F) in the air is 0.16 x cm2/s. The diffusion coefficient of oxygen in the air is 0.153cm2/s. The values are estimated as it is very difficult to directly measure a gas. On the other hand, the diffusion coefficient of the oxygen is strongly affected by temperature. For example, the viscosity of water at a temperature of 20 degrees Celsius is 1,002 centipoises, and at 40 degrees Celsius, the viscosity of the water is 0.653 centipoises.
The O2 diffusion coefficient in saturated air (15% Oxygen) is 5,700 to 10,800 times greater than in water (60°C and 20°C respectively). When oxygen is forced to diffuse through water saturated pores, this restriction on oxygen transport quickly leads to anaerobic conditions. Diffusivity increases with temperature and decreases with increasing pressure. While the diffusion coefficient can vary depending on the conditions in the gas phase, even more dramatic effects are seen when the oxygen must diffuse through water rather than air. In water at 60°C, the oxygen diffusion coefficient is approximately 4.8 x 10-5 cm2/sec, almost 4 orders of magnitude smaller than that in air. Because the impact of moisture is so dramatic, excess moisture is the most common factor leading to anaerobic conditions. Because oxygen diffuses so much slower in water than in air, excess moisture reduces oxygen penetration. This reduction occurs in two ways. First, because moist compost is hydrophilic (it loves water), water is strongly held to the surfaces of particles, so as water content increases the thickness of the aqueous film surrounding each particle increases. The second, closely related effect, is a matrix effect due to capillarity -- water fills the smallest pores first, and thus creates water filled zones between particles, which slow oxygen diffusion and result in anaerobic clumps.
Keywords: Lung; Alveoli; Fibrosis; Energy; Hydrogen; Melanin; Water
Introduction
The purpose of the respiratory system is to perform gas exchange. Pulmonary ventilation provides air to the alveoli for this fundamental gas exchange process. At the respiratory membrane, where the alveolar and capillary barriers meet, gases move across the membranes, while some atmospheric gases entering, the carbon dioxide leaving [2]. While the healthy lung efficiently exchanges respiratory gases, hypoxemia and hypercapnia indicate pathophysiology and failure of the lung to provide adequate gas exchange.
An understanding of how gases are transferred, and the causes of inefficient gas exchange are central to caring for patients with lung diseases. The structure of the lung is perfectly suited for efficient exchange of respiratory gases and has a pattern that repeated in numerous species. Through the airway and vascular trees, fresh gases and venous blood are delivered to and removed from a large alveolar capillary surface area. In an adult, inhaled air enters the trachea with a cross-sectional area of ≈3cm2 and is delivered to the alveoli with a surface area of ≈140m2, roughly the size of a tennis court [3].
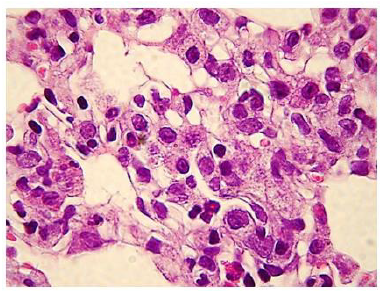
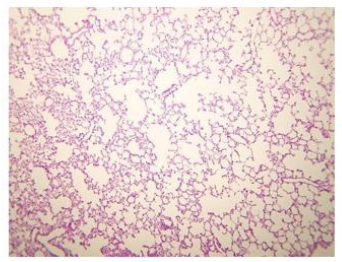
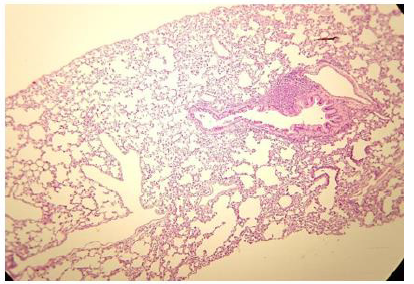
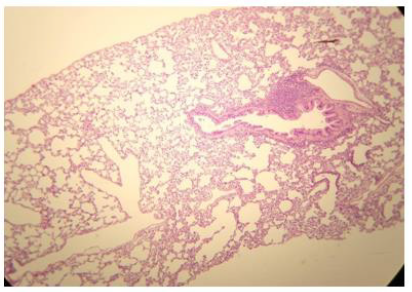
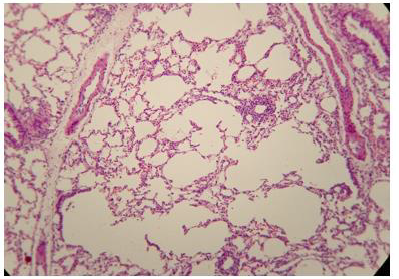
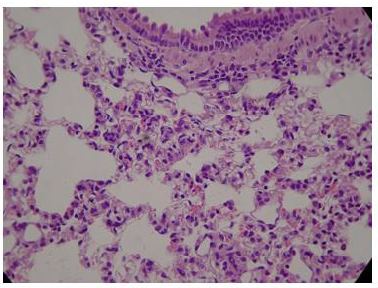
Figure 2: Histologic lung section, Wistar rat. Dyed with Hematoxylin and Eosin. The 100 X magnification allows to glimpse the very thin membrane (1 μm) that delimits the alveolar gas space from the blood capillaries lung compartments.
The pulmonary vascular tree begins as the main pulmonary artery and repeatedly bifurcates (fractal-like) into arterioles and capillaries that cover 85-95% of the alveolar surface. An exceptionally thin membrane of only 1μm [4] separates the alveolar gas and blood compartments, allowing gases to diffuse rapidly between them (Figure 2).
The partial pressure of O2 (PO2) in the alveolar gas (PAO2) is much higher than in the capillary blood but oxygen requires -characteristically- a high-pressure gradient to diffuse thereby O2 spreads almost passively from the alveolar space into the blood during passage through the capillaries. However, only ≈ one third of the contained oxygen in the alveolar space is absorbed. To comparative aims, ≈7% of Nitrogen is absorbed. No mechanism is known, in any species, that could increase the absorption of oxygen in the interphase alveoli/ blood, instead, in all living things [5] exist the carbonic anhydrase (carbonate dehydratase), an enzyme that speed up the passage of CO2 from the blood to the alveolar space between 10 and 100 times.
The catalyzation of carbonic anhydrase in our tissues is as following:

The first reaction is catalyzed by carbonic anhydrase and the second reaction occurs instantaneously [6]. The effect of carbonic anhydrase in the lungs is opposed:

In the lungs carbonic anhydrase converts bicarbonate to carbon dioxide, suited for exhalation. The explanation, so far; is the different pH levels found on them. Without the carbonic anhydrase catalyst, the reaction is very slow, in the range of 15 seconds; however, with the catalyst the reaction is 107 times faster [7].
The Uncertain Role of Oxygen
Basically, it must be understood that a gas travels from areas of high-pressure gradient to areas of lower pressure gradient. This concept apparently explains how oxygen travels through tissues. Furthermore, gases dissolve in liquids in proportion to their partial pressures (Henry´s Law). Theoretically, this is important because most oxygen inside the body is stored in fluid, such as blood. But the theory that the oxygen from the atmosphere is transported into the body through the lungs and then distributed by the bloodstream to all cells of the body, so, in turn, these cells, by combining oxygen with glucose, what is called oxidation or combustion; and finally gets energy. However, this sequence of events came across a barrier of contradictions and uncertainties, and so it is, that of the ≈7000 intracellular reactions described in the scientific literature, trying to explain this prevalent dogma; around 6800 are controversial [8] and worst, in many senses. Thereby, it’s not easy to explain this surprising level of disagreement. If we consider the work of Santoro Sanatoriums on fluctuations in body weight throughout the day, published in the year 1614 as the first published study about the metabolism of the body, then we have that after 400 years and after several million of published articles; we are worst that at first, because we do not understand yet, clearly, what happens within our body. So, going back to oxygen, and based on the finding of the unsuspected bioenergy role of melanin; it turns out that the body’s oxygen needs are any as it has been insisted to date, because the body does not need or want to burn or oxidize glucose to obtain energy.
It is not necessary because the energy it requires for metabolism, our body takes from light, thanks to the unsuspected intrinsic property of melanin to dissociate the molecule from water; neither should it be carried from the atmosphere into the cells through the lungs and blood vessels. The above for a simple reason: the eukaryotic cells are simple and simply capable of producing it on its own, by dissociating water. That is why we must rethink the physiology of breathing. The main function of the lungs is gaseous exchange, but the basic function is to expel CO2, not oxygen absorption. The absorption of gases from the atmosphere is secondary, and it is logical because they have always been present. But the amount of oxygen that is absorbed is only one third of what is found in the lung, this is 7%, and the same goes for nitrogen, as 7% is also absorbed.
The amount of oxygen normally absorbed through the lungs could not meet the expenditure required by a biological system that based its energy source on glucose oxidation (combustion). The presence of high oxygen levels in some tissues containing hemoglobin and myoglobin, it was because both myoglobin and hemoglobin can irreversibly dissociate the water molecule [9] like chlorophyll in plants. Therefore, in their biology they are always associated with oxygen and water, but because they are constantly producing it arising from water dissociation.
The Absorption of Oxygen by the Lungs
Since the studies of John Haldane (1892-1964) geneticist and evolutive biologist, he concluded that the exchange of oxygen between the atmosphere and blood through lungs could not be adequately explained only by diffusion [10]. In the description of his interesting experiments, it is possible to find hints that went unnoticed of the surprising human body’s ability to take energy from sunlight. Haldane and Lorrain Smith measured oxygen levels in the blood in dogs and other animals through the carbon oxide method. Once they did the procedures and slaughtered the experimental animal, the percentage of carboxyhemoglobin in the experimental subject’s blood was determined against a previously standardized carmine solution.
Literally, Haldane wrote: “In carrying out the titrations we avoided bright light, which, from the experiments given in our previous paper, might have appreciably affected the results”. Despite the detailedness of his descriptions Haldane does not delve into the reason for this observation. Another interesting observation of Haldane was as follows: “We found, after making several experiments, that it was necessary to be careful to keep up the body temperature of the mouse. We kept the lower part of the bottle containing the mouse in a bath of warm water (25°C-30°C).” By the way: the best range of environment temperature at which the generation and distribution of energy from melanin is optimal is between 20 °C and 30 °C. “The metabolism of the mouse is also about ten times as great per unit of body weight as that of the man” This can be explained by Kleber’s Law or E= M3/4, which, in turn can be explained, finally by the unsuspected intrinsic property of melanin to dissociate the water molecule [11].
“After exposure to a cold atmosphere for a short time, their body temperature begins to fall. The result invariably was that the oxygen tension went down to about 15%. The uniformity of the values observed here suggested that the cold had nearly paralyzed the mechanism of absorption, and that the tension observed was approximately that due to diffusion from the alveolar air.”
The generation and distribution of energy is significantly diminished with cold weather, the example is wintertime that induces hibernation in plants and animals. Notice the word invariably and happen in the animals of experimentation all. The explanation is that melanin is present in all living things, with the same physical-chemical properties and the same function energy production. “In all the animals observed the oxygen tension of the arterial blood as it leaves the lungs is considerably higher than the alveolar oxygen tension, while in man and small birds, and often in other animals, it is very much higher than that of inspired air (19.7% allowing for aqueous vapor).”
The remarkable uniformity observed in several fundamental parameters between the different species, underlines the presence of melanin in all of them, since their physical/ chemical characteristics as well as function are the same in all living beings: production of Energy. “In this series of experiments, which were made on mice, we investigated the effect of the lack of oxygen which must arise in the body when a large portion of the hemoglobin is saturated with carbonic oxide. The results (Table 3) show that when more than 60 % of the hemoglobin is saturated, and the other respiratory conditions are normal, the tension of oxygen begins to rise very markedly.”
The generation and distribution of energy from melanin is significantly stimulated when 60% of hemoglobin is saturated with carbon oxide. It is a coherent physiological response if we interpret it based on the now unknown ability of living beings to capture energy from sunlight. “On the other hand, we found that birds were unable to survive after about 60% of their hemoglobin had been saturated. They usually became restless and died suddenly just after some unusual exertion.”
When saturation is greater than 60%, then the effect begins to be opposite, because it is then that the intrinsic ability of melanin to dissociate the molecule from water begins to gradually depress itself. “In the case of men poisoned by gradual absorption of carbonic oxide after explosions in coal mines, the highest saturation observed was 83% (v. Haldane, Report on the causes of death in Colliery Explosions, 1896, p. 40), but in many cases the saturation found was considerably less.”
If hemoglobin oversaturation is gradual, melanin can respond to greater challenges, as melanin is increased to punishment. When oversaturation occurs suddenly, melanin has less time to compensate. “A very remarkable fact brought out by these experiments is that, provided the natural fall of body temperature is avoided, the mouse can still live and retain consciousness and the power of creeping about after as much as 84 % of its hemoglobin has been thrown out of action by saturation with carbonic oxide.” Under adequate conditions, the generation and distribution of energy coming from melanin, can keep the organisms alive even hard environmental conditions. “Carbonic acid does not, therefore, appear to act as a stimulus to the absorption of oxygen by the lungs.”
Many poisons, in small doses, act as a stimulant of melanin activity to dissociate the water molecule. “The absolute difference between alveolar and arterial oxygen tension increases, but the relative difference remains about the same.” The physical and chemical stability of melanin as well as its behavior is surprising. Proven 170 million years [12]. “The degree of absorptive activity on the part of the lung depends on the supply of oxygen to the tissues of the body generally and not merely on the oxygen tension already reached by the blood on its way through the lung capillaries. “
Oxygen levels in tissues depend on the dissociation of the water that each cell performs at its own, the oxygen levels in the blood does not matter much. “The oxygen tension of the alveolar air was calculated on the assumption that the ventilation of the lungs was about thrice as great as usual, the respiratory exchange of the animal remaining about the same. It seems not improbable however that the increase in ventilation of the lungs is over-estimated and that the alveolar oxygen tension was lower.”
Notice: the increase in ventilation of the lung is over-estimated. It is a collective error that the frequency of ventilation is a determining factor in the oxygen levels in the blood, but it is not the case because these levels depend mainly on the dissociation of the water molecule that takes place in each cell of the body, thanks to melanin. “Thus, in the last experiment in the table the frequency of respiration had fallen to 120, and the individual respirations did not appear to be much deeper than usual.” Increasing the frequency of breathing has no greater effect on the oxygen saturation of the blood, since such saturation is determined by oxygen levels from melanin-by the dissociation of water. “The fact that the arterial oxygen tension was proportionally so much higher than that of the inspired and alveolar air proves that fall in the arterial oxygen tension beyond a certain point directly or indirectly stimulates the lung to greater absorptive activity.” It is not proven, and it is not possible to stimulate the absorption of oxygen by the lung in a significant way. The answer is the automatic adaptation of melanin, this is the major or lesser dissociation of the water molecule depending on the circumstances inside of the body and those of the environment. “The death of the animal is evidently due in some way to the fall in the oxygen tension of the blood, and not to mere diminution in the percentage of oxygen present in it, since with a tension of 7 or 8% of oxygen (the minimum observed) the hemoglobin would still, according to Haefner, be 96% saturated with oxygen.” Hemoglobin can irreversibly dissociate, such as plant chlorophyll, the water molecule. It is no by chance that the great resemblance between the chlorophyll and hemoglobin molecules.
“The experiments in (Table 3). show that a mouse can still live and retain consciousness and sufficient power of movement to enable it to creep about, when its hemoglobin is only 16% saturated with oxygen, provided the oxygen tension of the blood is at the same time high.” If the ratio of the inspired oxygen (from the atmosphere) to the oxygen produced by the melanin is maintained, the system remains viable. “The behavior of carbonic oxide in the body differs from that of oxygen in as much as the latter is withdrawn from the blood to be consumed in the metabolism of the body, whilst the former does not disappear in this way, and therefore cannot be affected by changes in the circulation.” If oxygen is absorbed by the body’s tissues as blood circulates through the body, how does oximetry in the fingers draw the same results as in the toes? “It is thus evident that the blood in the veins must be at least 15% saturated with oxygen even in the very last stages of asphyxia. We may therefore assume that at least 4% of oxygen is present in the arterial blood, although the blood-pump may give no oxygen, even in the earlier stages of asphyxia.” If the heart is not pumping, the only possible source of oxygen is the dissociation of water by melanin. “The lowest pressure with which we found it possible to work was in the case of mice about 25% of an atmosphere. Birds suffered much more than mice from low pressures. Even at a pressure of 34% of an atmosphere one of the birds died after 28 minutes. The results shown in the table entirely confirm the observations given in the section on experiments with air partially deprived of oxygen, since they show again that up to the time of death the tension of oxygen in the arterial blood leaving the lungs is high enough to saturate the ha3moglobin almost completely and is far above the alveolar tension.”
The experimental animal dies despite the apparent complete saturation of hemoglobin -on oxygen - because oxygen has nothing to do with energy production. Oxygen is toxic, but it is a bad necessity because it has always been present in the equation. The real reason is that if oxygen levels in the environment are not natural range of 21 or 22%, then CO2 cannot spread as it should be, and thus, the generation and distribution of energy coming from melanin turns quite sensitive to higher or even normal levels of CO2.
“To test the truth of these results Stroganov used the method, suggested by Hoppe-Seiler, of examining the spectrum of the blood while still circulating in the jugular vein of an animal with the trachea clamped. He never failed by this means to find in the asphyxiated animal’s blood the double bands of oxyhemoglobin, and that for a long time after the cessation of respiration. Even in the veins the bands remained visible up to the point of complete cessation of the heartbeat, although after this they quickly disappeared.”
The main part of Oxygen levels come from the dissociation of water that occurs inside melanin. A minor part comes from the air lungs or breathing. Notice the phrase: “and that for a long time after cessation of respiration.”
“In order to obtain a rough idea of the minimum percentage of oxygen which must have been present even in the venous blood in these experiments we took a test-tube containing a very dilute solution of completely reduced hemoglobin and placed between it and a gas-flame another test-tube of equal size containing oxyhemoglobin in varying dilutions. We found that when the oxyhemoglobin solution was less than 1/6 the of the strength of the reduced hemoglobin solution it was no longer possible, even with the most favorable concentrations, to see the oxyhemoglobin bands through the band of reduced hemoglobin.”
Reproducing irreversible dissociation of water that occurs in hemoglobin, or myoglobin in a test tube is not easy in any way. “We have inferred from these experiments and those in the previous section that in asphyxia from atmospheres at low pressures or partially deprived of oxygen the symptoms are due, not to lack of oxygen in the blood, but to the diminution in the oxygen tension.”
Oxygen stress is important for normal CO2 mobilization. If the diffusion of CO2 is distorted, the generation and distribution of energy from melanin begins to depose rapidly, so a widespread failure begins to occur that leads rapidly to the death of the experimental animal.
“It seemed to be invariably the case that if the pressure or percentage of oxygen was reduced too rapidly symptoms of asphyxia were apt to appear. Several animals nearly died in this way, although after a time they were able to live perfectly well in the same atmosphere, and the blueness of the skin, which was at first evident, disappeared.”
Choking symptoms appear because CO2 levels begin to rise because of abnormal oxygen levels that not fitting into the equation, and disturbance in normal CO2 dissipation present in tissues results in the generation and distribution of energy from melanin begin to affect rapidly body functions.
If the affected organism has enough time to adapt to the new conditions of environmental oxygen, then melanin gradually adapts the processes related to energy generation and distribution so that they are sufficient to sustain the life of the experimentation subject as appropriately as possible. We must keep in mind that melanin is growing to punishment.
“It seems not unlikely that the importance to the animal of the attainment of a high oxygen tension in the blood passing through the lungs is in some way connected with the large amount of oxidation which, according to the very striking experiments of Bohr and Henriques’, occurs in the lungs themselves. The experiments of the latter observers, showing how very slight an effect on the metabolism is produced by almost completely blocking the circulation, seem also to point in the same direction as our experiments on the effect of very high saturation of the arterial blood with carbonic oxide.”
Haldane almost completely blocks circulation and tissue metabolism is almost unaffected. If we start from the unexpected ability of living beings to capture energy from light, the explanation is obvious, but if we insist on considering the glucose as a source of energy then we immerse ourselves in a sea of controversies that takes centuries.
J Haldane and JL Smith
In Bright Weather the Room Was Also Partially Darkened During the Titration
“We have not yet determined the dissociation curve of carboxyhemoglobin in the absence of oxygen, but we may mention here the results of one or two preliminary experiments in which mixtures of carbonic oxide and hydrogen were employed. Thus, we found that at 37°C, and with a tension of .0044% of carbonic oxide, hemoglobin solution after 20 minutes’ shaking became 80 % saturated. At 15°C, and with .0047% of carbonic oxide the hemoglobin became 98% saturated. The hydrogen employed in these experiments was, however, not quite free from oxygen, and the shaking was probably not continued long enough, so that the saturation obtained at 37° can only be regarded as a minimal one.” Until Haldane added hydrogen, the saturation of hemoglobin in the test tube and with agitation, reached 98%. In two similar previous experiments, without hydrogen, saturation only reached 56.5% and 48.2% after 84 and 95 minutes of uninterrupted agitation.
Haldane does not explain why he add hydrogen nor because thus the saturation of 98% was reached.
Haldane’s chief conclusions in that article were as follows:
“In the animals investigated the normal oxygen tension in the arterial blood is always higher than in the alveolar air and is in some animals even much higher than in the inspired air. The absorption of oxygen by the lungs thus cannot be explained by diffusion alone.” The article was published in 1897, and since then they had detected that the single diffusion did not explain blood oxygen levels.
“Fall in the body temperature causes a marked fall in the oxygen tension of the arterial blood.” The generation and distribution of energy from melanin is significantly affected with extreme climates, both cold and heat. And the most interesting thing was the uniformity of the fact, occurred in all the species in which Haldane made experimentation; and the explanation is that the basis of life is the same in all living things: melanin.
“Increase in the oxygen percentage of the alveolar air causes an almost proportional increase in the oxygen tension of the arterial blood, so that active absorption still proceeds in atmospheres richer in oxygen than atmospheric air.” Haldane detected the body’s ability to dissociate the molecule from water but interpreted it as such. He thought that there was something like active oxygen absorption, which to date, no one has been able to prove in any specie.
“Diminution in the oxygen percentage or tension in the alveolar air causes a fall in the oxygen tension of the arterial blood; but want of oxygen, whether produced by carbonic oxide poisoning, by diminution in the oxygen percentage of the air, or by diminution in atmospheric pressure, causes a marked increase in the relative excess of arterial over alveolar oxygen tension: hence want of oxygen acts as a stimulus to absorption of oxygen.” What Haldane calls “oxygen absorption” is in fact the ability of organisms to take energy from light by dissociating the molecule from water, which is not a linear process but varies according to the factors of the environment both internal as well as external. But Haldane’s observation is very accurate, because the small doses of toxins, and the conditions that Haldane refers to, within certain ranges, induce a response of melanin in which it increases its activity - of dissociation of water-, resulting in oxygen levels inside the body rising. Of course, if doses of toxins, poisons, or if environmental conditions relating to oxygen exceed certain limits, the effect is opposite, and the production of energy by melanin starts to collapse and can go all the way to cease completely, to which death is in minutes
“The symptoms caused by diminution in the oxygen tension of the air breathed are due to fall in the oxygen tension reached by the blood in the lungs, and not to diminution in the quantity of oxygen carried by the blood from the lungs.” The inquisitiveness of Haldane’s experiments is surprising. Well, he could detect clearly that oxygen changes in the atmosphere don’t determine blood oxygen levels. Haldane was no further, and his remarkable findings explained them as oxygen absorption. We now know that the body does not actively absorb oxygen and that our body is able to produce it on its own, by dissociating the water molecule, which produces molecular oxygen and molecular hydrogen. As a result, Haldane was only able to reach 98% saturations in hemoglobin when he added hydrogen to the experiment. Which is logical because hydrogen is the main energy carrier in the entire universe [13]. It would have been interesting to know the reasons why Haldane added hydrogen only in some experiments, and because he didn’t consider the role of the hydrogen during the analyze of the results.
So, we could redefine the function of blood in transporting the CO2 from the tissues to the lungs, where it would be expelled into the atmosphere, and the transport of metabolic intermediaries. concluding blood cannot carry energy. The expulsion of CO2 is of paramount importance since, in average, our body produces 900 grams of carbon dioxide each 24 hours, this is 77.7777778 grams per kg, 0.07777778 grams per gram of tissue; thereby, each cell in the body produces about 7e 13ml per minute of CO2 [14]. This CO2 must be quickly eliminated from to prevent acidosis. CO2 is 20 times more soluble than oxygen, and therefore diffuses quickly. Likewise, while oxygen requires a high-pressure gradient to diffuse, CO2 requires only a small pressure gradient.
The Importance of Lung Tissue Integrity in Gas Exchange
Despite Haldane’s extraordinary work, the currently published articles insist that the single diffusion explains the noticeable difference between alveolar oxygen levels and blood oxygen levels. Let´s see some examples:
The capillaries of the lung deliver mixed venous blood with a low partial pressure of O2 (PvO2). The partial pressure of O2 (PO2) in the alveolar gas (PAO2) is much higher than in the capillary blood and O2 diffuses passively from the alveolar space into the blood during passage through the capillaries. The concept of simple diffusion as a simple explanation for the noticeable differences between oxygen levels in alveoli and blood, are detrimental to the care of patients. The hemoglobin molecules have no impact on the partial pressure of oxygen. However, some references are contradictory: The amount of O2 carried in the blood is determined by the hemoglobin concentration, the fraction of hemoglobin binding O2 and the PaO2. The end-capillary blood O2 content (CecO2), rather than the PecO2, from different alveolo-units are additive.
Exercise increases the amount of O2 extracted from arterial blood in the systemic circulation, which tends to reduce PvO2. Therefore, more O2 needs to be taken up in the lung to reach normal oxygenation of arterial blood. Exercise also increases pulmonary blood flow, which shortens the time that the blood is exposed to alveolar gas. The combined effect is that more O2 needs to be taken up in less time. However. Haldane wrote that breathing frequency has not a significant effect on hemoglobin saturation. With the analysis of the mechanisms of gas exchange in the lung is happening the same as with the study of biochemical processes related to metabolism: they all tell a different story.
So, we will limit ourselves a few precepts that are demonstrable with some ease:
In acute respiratory failure syndrome, gas exchange is characterized by severe hypoxia due, among other things, to the loss of gas exchange areas due to anatomical alterations characterized by edema, hemorrhage and cell infiltration which further hinders gas exchange and mainly the efficient outflow of CO2. Remember that, according to Haldane, alveolar oxygen pressure is not the main determining factor in relation to blood oxygen levels. According to our work, the oxygen we find in the tissues comes, in reduced portion, from the outside, but, in its largest portion, it comes from the cells themselves that possess melanin and are therefore able to dissociate the water molecule producing oxygen and hydrogen, molecular both.
The generation and distribution of energy by melanin is an astonishingly accurate process, and the most surprising thing is that it is the same process since the beginning of time, because despite millions of years of evolution, it has not changed, it cannot change, not to change. In fact, acute respiratory insufficiency syndrome is due to an imbalance of the fundamental bioenergy function of melanin, and which is conditioned by contaminated water, contaminated air, pesticides, herbicides, fertilizers, metals, plastics, solvents, industrial waste, emotional tensions, alcohol, extreme climates, dehydration, etc. When the generation and distribution of energy is altered, the body began to disorganize and eventually appears what we call diseases, which do not actually exist as such, everything is reduced to an imbalance between mass and energy. So it is, that the human body ignores the names of diseases.
Then, when we unbalance the so accurate processes of generation and distribution of energy by means of toxic agents, for example arsenic; then the processes of the body begin to disorganize. When the imbalance is acute, characteristically there is edema and bleeding. When the imbalance is chronic, then there is fibrosis and uncontrolled mitosis. To avoid controversy, we designed an experiment with the purpose of demonstrating that the mechanism of action of some toxics, such as arsenic, is because it significantly alters the generation and distribution of energy emanating from melanin. And at the same time, we could demonstrate the therapeutic effect of a new drug: QIAPI 1©, which protects and improves the mechanisms related to that generation and distribution of energy.
Material and Methods
The Pentavalent As is well absorbed by the GI tract, initially located in blood, attached to globulin, redistribution occurs within 24 hours of the liver, lungs, intestine, and spleen. The As replaces bone phosphorus, where it remains for 30 years or more. 3 groups of Wistar rats, adults, males, each group of 10 individuals. The first group was controlling:
The second group was exposed to the arsenic (As) in the water, and the third group was as + QIAPI1©.
The duration of the experiment was 4 months, during which they were administered the As, As and QIAPI1© to the exposed groups, and normal water to the control group.
Results
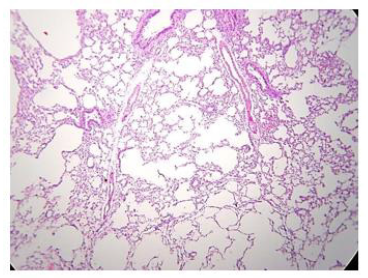
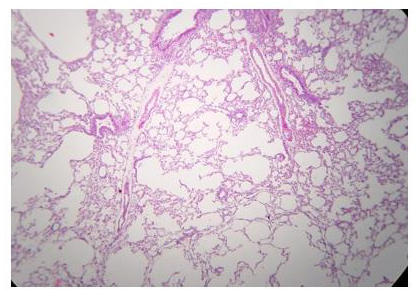
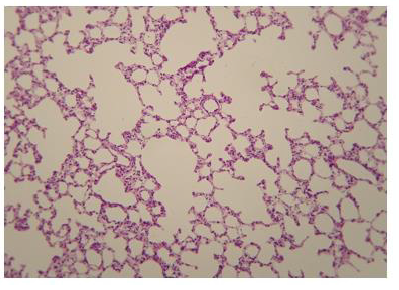
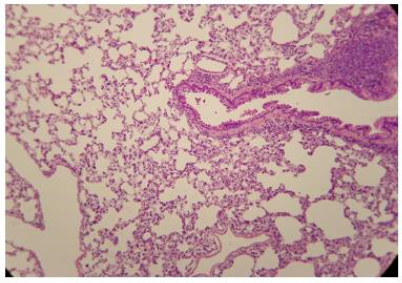
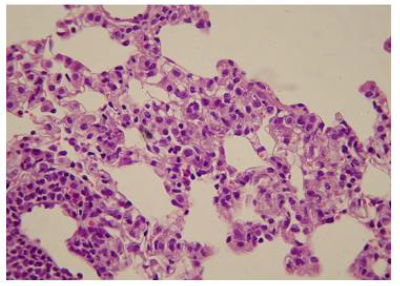
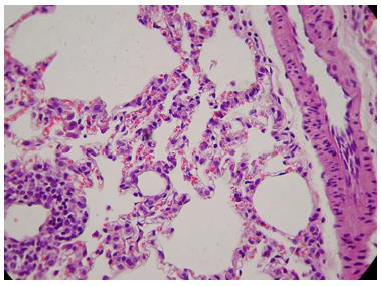
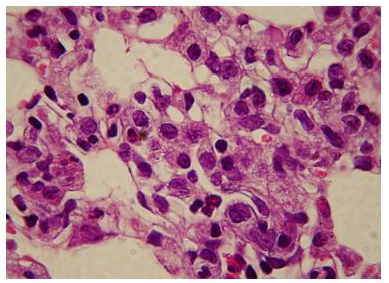
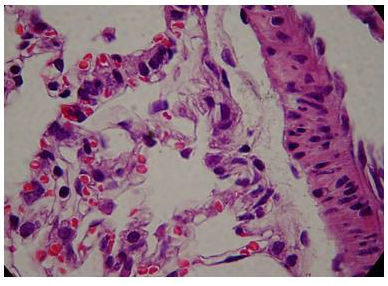
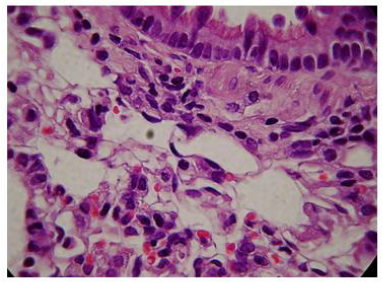
The administration of penta-valent Arsenic in drinking water (ad libitum) caused significant lung damage marked by edema, vasodilation, thickness of the alveolar wall, and infiltration of inflammatory cells mainly in the interstitial space and eventually in alveolar space. All these Arsenic induced changes were substantially less in animals treated simultaneously with QIAPI 1©, particularly the virtual absence of interstitial edema. (Figures 3-16)
Comments
The Bioenergy role of melanin is validated by the histological sections showing the detrimental effect of pentavalent Arsenic and the beneficial effects of QIAPI 1©, the latter reduced significantly histological signs of lung inflammation induced by Arsenic. It is important because impaired alveolar fluid clearance is associated with worse survival [15] and increased development of fibrosis [16]. Increased concentrations of nitrate and nitrite in edema fluid samples of patients with severe pneumonia were associated with slower rates of alveolar fluid clearance [17].
Conclusion
The lung is a shock organ, and its important role in CO2 management makes it a vital organ, as elevated levels of CO2 in the blood can cause death in less than a minute. In the case of pneumonias of viral or bacterial origin, or even fungi, the story does not begin with the simple presence of the etiological agent, which is replicated explosively causing severe pneumonia that can lead to the death of the individual quickly. Fungi, viruses, or bacteria do not attack us, because we have shared the planet since the beginning of time.
What happens, is that when the microenvironment of the bacteria becomes hostile, for example by the presence of pesticides, herbicides, fertilizers (e.g. nitrates and nitrites), plastic metals, industrial waste, alcohol, solvents, abuse drugs, etc., which also disturb the delicate balance between mass and energy in the human host, is when the bacteria respond aggressively, but when the situation improves in the disease, that is: the balance between mass and energy recovers, then the microenvironment of the bacteria becomes friendly and the problem is finished because the behavior of the infectious agent became normal again.
Then, infections do not lead to the imbalance of the person, instead the person is previously unbalanced by the factors mentioned above, which in turn keep the air and water continually contaminated. So, a person who is not on balance is more likely to have pathogens exhibit abnormally aggressive behavior because contaminants also have an impact in the infectious pathogen agent itself due to their biochemical have resemblance with our biochemical conformation -ATP, glucose, RNA, DNA, NADH, glucose, amino acids, oxygen, peptides, etc. And it happens not only in infections but in several other diseases in spite to be classified as metabolic or degenerative, because the balance between mass (from glucose) and energy (from melanin) is so strict that it is analogous since the beginning of time, and after millions of years of evolution has not changed. When a person is in imbalance, the functions and even anatomy of the body begin to disorganize, as the body’s metabolic processes are very accurate, astonishingly accurate. And it is when what we call diseases appear that do not actually exist as such, but all of them are reduced even an imbalance of the fundamental functions of life. It is no coincidence that the most severe pneumonias (atypical or not) are observed in countries with cold climates, as the intense cold tends to unbalance both plants, animals, even bacteria, fungi, and viruses; as well as more polluted countries, for example with nitrates widely used as fertilizers. Concentrations of nitrate below 0.3mg N/l are natural or background levels for most European rivers though for some rivers levels of up to 1mg N/l are reported. Concentrations of nitrate above 7.5mg N/l are of relatively poor quality and exceed the guideline concentration for nitrate of 5.6 mg N/l as given in the Surface Water for Drinking Directive (75/440/EEC) [18].
Metabolism means continuous change. Energy means everything that produces a change. Therefore, the continued changes of the metabolism, require energy in form constant, continuously, night and day. The ancient and wrong idea of that the cell interacted with the environment, obtaining energy by means of the combination of the oxygen with the glucose (Oxidation or graduated combustion), probably born from the works of Lavoisier and Priestley. Some examples of discrepancies between theory and facts about glucose as source energy are the following:
The very structure of the mitochondrial membrane supposes a strong discrepancy in the biochemical/biomechanical model of the electron flow is, which is impermeable to the passage of the NADH reduced by the extra-mitochondrial glycolysis. The active center of the electron-carriers are their prosthetic groups consisting almost exclusively of reactive transition metals. According to this model, the electrons are passed on from one metal center of an electron carrier to the next by rotatory and translatory motions and so lose their initial energy. Furthermore, one still cannot explain the fact that every NADH donates two electrons, for instance, while every O2 molecule needs four electrons to generate water, this is: 2H2O.
The corpuscular concept of the electron flow would therefore demand the existence of several electron collecting and distributing points along the respiratory chain, where differences in the number of electrons must be balanced. Until the electrons are eventually passed on to O2, the electron-transferring potential serves to supply three oxidative phosphorylation complexes through the reduction of NADH respectively FADH2. In consequence, a major part of the released energy can be passed on directly instead of being lost to the environment in the form of heat. The corpuscular concept starts from the assumption, though, that the reaction takes an indirect course and hydrogen atoms are divided into hydride ions (H- and H+), that is, into protons and electrons, which – but for a few incidental encounters - only combine again at the very end of the respiratory chain. And that is what the biochemical/biomechanical model is all about, regarding the processes in the cell as random and chaotic occurrences without any synergetic precision. As mentioned above, the electrons are thought to be transferred to the respiratory chain because of incidental encounters, which are explained in terms of vibrations and rotatory motions of the parameters involved that go as far as the quantum-mechanical tunneling of membrane barriers. Furthermore, it was yet not deemed necessary to make the wellordered electron transfer postulated in the biochemical models solely contingent on the specificity of the functional correlation between the component parts of the respiratory chain.
Contrary to the random and chaotic organization of the mitochondrial energy transfer assumed in the classic corpuscular model, functional movements and changes in the cell take place in a highly ordered way. Otherwise, the feedback control system cell and the entire human organism would not be able to exist in the end. This control principle is only functioning properly, however, if the highly structured processes can be attributed to long-range correlations between the various components and systems, which far exceed the radius of action of chemical forces. As this in turn suggests the vibrational aspect of matter, one cannot help disassociating oneself from an exclusively molecular point of view. Regarding the theoretical model, the mitochondrial electron flow thus turns out to be a wave (of Hydrogen) instead of the classical molecular shifting of particles. The correlation between energy transport (radiation) and order (molecular structure) becomes apparent when structurally bound energy is released in consequence of the breakdown of compounds in molecular units or released energy manifests itself again in structural form.
There is, in other words, a constant, consistent interactive relationship between energy and structure, a circumstance that is not only in much better accordance with the modern physical quantum models, but also explains and describes the aspect of the electron flow involved in the mitochondrial energy transfer in a way that is much more logical than the prevailing concepts, which are rather hard to grasp. Essentially, chemical reactions in the cell consist of the division or combination of cellular reaction partners. Examples for this are the oxidation of foodstuffs in the citric acid cycle and the production of ATP in the respiratory chain. If reactions are to take place at all, the components involved must receive enough kinetic energy to meet. Furthermore, at least one of the reaction partners must be activated – and be it only for a short time - to alter its electric charge, for instance, before it can combine anew.
These inconsistencies and the reasons given above clearly suggest that the mitochondrial energy transfer is associated with radiation phenomena more than corpuscular phenomena. Thereby, inside the cell, melanin constitutes the fundamental source of wearable energy through the dissociation and re-forming of the water molecule. The hydrogen is the main carrier of energy in the whole universe, our body does not can be different. Our body could obtain hydrogen from water if it were able to dissociate the water molecule. During an observational study about the morphological characteristics of the optic nerve´s blood vessels and its relationship with the three leading causes of blindness, we found, after reviewing ocular fundus studies of ≈6000 patients (1990-2002), that melanin has the unsuspected intrinsic ability to dissociate the water molecule [19].
In Plants (Chlorophyll)

In Humans (Melanin)

The energy that emanates from the melanin in the form of molecular hydrogen and electrons of high energy can explain all the cellular functions.
Acknowledgment
This work was made possible thanks to the unrestricted support of the Center for the Study of Human Photosynthesis, S.C.
References
- Gutknecht, John Bisson, MA Tosteson FC (1997) Diffusion of Carbon Dioxide through lipid bilayer Membranes. J Gen Physiol 69(6): 779-794.
- Petersson J, Glenny RW (2014) Gas exchange and ventilation-perfusion relationships in the lung. Eur Respir J 44(4): 1023-1041.
- Gehr P, Bachofen M, Weibel ER (1978) The normal human lung: ultrastructure and morphometric estimation of diffusion capacity. Respir Physiol 32(2): 121-140.
- Prefaut C, Durand F, Mucci P (2000) Exercise-induced arterial hypoxaemia in athletes: a review. Sports Med 30(1): 47-61.
- Ghandour MS, Parkkila AK, Parkkila S, Waheed A, Sly WS (2000) Mitochondrial Carbonic Anhydrase. Proc. Natl. Acad. Sci. USA 75(5): 2212-2220.
- Breton, Sylvie (2001) The cellular physiology of carbonic anhydrases. JOP. Journal of the Pancreas 2(4 Suppl): 159-164.
- Lindskog S (1997) "Structure and mechanism of carbonic anhydrase". Pharmacology & Therapeutics 74(1): 1-20.
- Stobbe MD (2020) The road to knowledge: from biology to database and back again. Dissertation and Thesis, 2012. Faculty of Medicine, University of Amsterdam.
- Herrera, Arias AS, MPS (2013) The Odyssey of Atmospheric Oxygen in their futile attempt to Reach the interior of the cell Int J Health Innovation.
- Haldane, Smith, Lorrain J (1897) The absorption of oxygen by the lungs. J Physiol 22(3): 231-258.
- Aliev Gjumrakch, Solis Herrera Arturo, Li Yi, Yury G Kaminsky, Nikolay N. et al. (2013) Human Photosynthesis, the ultimate answer to the long term mystery of Kleibers Law or E = M3/4. Open J Psychiatry 3(4): 408-421.
- Glass Keely, Ito Shosuke, Wilby Philip R, Takayuki Sota, Atsushi Nakamura, et al. (2012) Direct chemical evidence for eumelanin pigment from the Jurassic Period. 109(26): 10218-10223.
- Herrera Arturo S, Esparza MCA, Aliev Gjumrakch, Andrey A Zamyatnin, Gjumrakch Aliev, et al. (2015) Beyond Mitochondria, what would be the energy source of the cell? CNS Agents in Med Chem 15(1): 32-41.
- Bottrell, John (2020).
- Matthay MA, Robriquet L, Fang X (2005) Alveolar epithelium: role in lung fluid balance and acute lung injury. Proc Am Thorac Soc 2(3): 206-213.
- Brody AR, Bonner JC, Overby LH, Badgett A, Kalter V, et.al (1992) Interstitial pulmonary macrophages produce platelet-derived growth factor that stimulates rat lung fibroblast proliferation in vitro. J Leukoc Biol 51(6): 640-648.
- Zhu S, Ware LB, Geiser T, Matthay MA, Matalon S (2001) Increased levels of nitrate and surfactant protein a nitration in the pulmonary edema fluid of patients with acute lung injury. Am J Respir Crit Care Med 163(1): 166-172.
- European Environment Agency. (2020) (WEUO2) Nitrogen and phosphorous in rivers. Indicator Fact Sheet.
- Solis Herrera A, Arias Esparza MC, Solis Arias RI, Paola E Solís Arias, Martha P Solís Arias, et al (2010) The unexpected capacity of melanin to dissociate the water molecule fills the gap between the life before and after ATP. Biomed Res 21 (2): 224-227.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.